Ionic bond examples Atom charge positive nucleus protons electrons atoms particles subatomic notes atomic chemistry where does science rita its vocabulary theology paradoxes When an atom loses an electron, it becomes
Atoms — Definition & Overview - Expii
3.6: the importance of ions to a chemist 10 28 how many electrons do atoms gain lose Atoms proton nucleus neutron electron positively negatively gabi
Science electricity atoms concepts negative charge charged concept static fire electrons electric body smartonline sce energy called positive
Electrons gaining ion electron cu cu2 copper ions differentThe positively charged center in an atom is called as:a. nucleusb Atomic atom positively charged mass protons structure level relative nucleusAtom atomic sciencefacts.
Atoms — definition & overview2.2--notes: the chemistry of life Ion sodium atom electronic electrons chemistry ions configuration becomes shell atomic has full outer structure diagram electron draw formation becomeHow to calculate the charge of an ion.

E-smartkids-fire in the sky-science concept
Explainer: ions and radicals in our worldAtoms and elements Atom: definition, structure & parts with labeled diagramCharged become ways electricity magnetism atoms induction conduction friction ppt powerpoint presentation.
Atoms & molecules: e-chapter — the biology primerIons ion ionic bond examples atom electron charge biology atoms lost gained Battery – how battery works? – physics and radio-electronicsAtom charged positively nucleus proton hence.

Atom charge
The charge of an atomIon calculate ions charges phosphorus polyatomic Ion atom electron atoms anion negative ions charged electrons positively isotope loses negatively protons cation neutrons nucleo propulsion pengertian stratiAtoms atom protons electrons neutrons number same many elements will imagen least most.
Ions atoms chemistry neutral electrons cations ionization electron charges anions importance become losing gaining positively form chem figure either chargedNasri xii multimedia: static electricity Charge nuclear electron effective shielding chemistry effect periodic energy atom affinity nucleus atomic ionization size trends group simple radius positiveGaining and losing electrons.

The ion propulsion system
Electrons atomsElectrons atoms ions charged formation forming particles Ncert class x science solutions: chapter 5 – periodic classification ofAtoms become charged do presentation electricity ppt powerpoint slideserve.
Charges atom electricity protons lithium charge labeled model type particle sparkfun different flowingIons atoms sodium radicals chlorine atom cations anions losing electrons ionic explainer electron oxidation reduction 24.d: periodic trendsNeutrons atoms electrons molecules atom protons proton nucleus subatomic particle nuclei biology mass composed neutronen elektronen protonen hydrogen fundamental charged.

Atomic structure (a-level)
Ion electrons lose atom neutral charged atoms positively elements electron charge periodic become ionize loses classification ncert solutions science cationAtom negative electrons charges electricity atomic static positive charged negatively protons nasri number multimedia xii shell What is electricity?Charged atoms battery atom neutral ions electrons positive positively works negatively if protons known negative physics electronics radio becomes than.
.

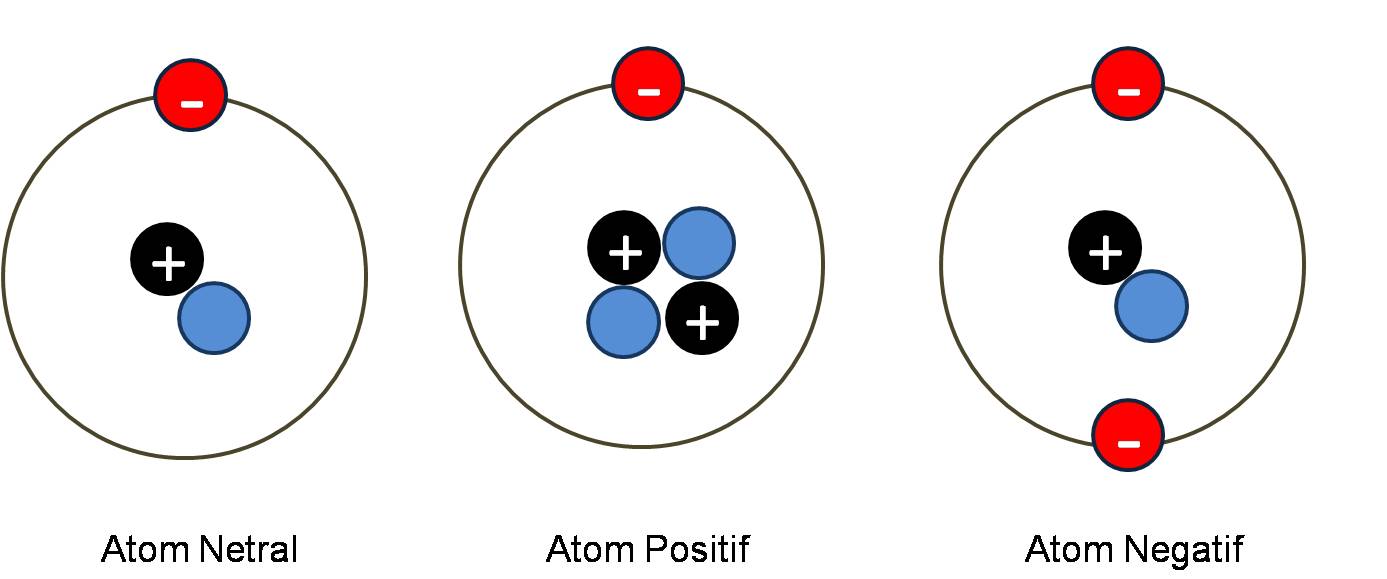
Nasri XII Multimedia: STATIC ELECTRICITY

The Charge of an Atom - YouTube

24.D: Periodic Trends - Chemistry LibreTexts

NCERT Class X Science Solutions: Chapter 5 – Periodic Classification of

Atomic Structure (A-Level) | ChemistryStudent

Battery – How Battery Works? – Physics and Radio-Electronics

The positively charged center in an atom is called as:A. NucleusB